Developing non-invasive diagnostic devices, whether for use in clinical settings or for patient use at home, is a core part of patient health. From lab-on-a-chip diagnostics to EWOD microfluidic devices, we have worked with clients to solve some of the complexities of bringing diagnostic devices to fruition.
We have in depth knowledge and experience in in-vitro and in-vivo device development that need to adhere to Federal and European medical regulations.
Innovation in this industry knows no bounds and we are excited to be part of this journey with our clients.

In vitro diagnostics
Springboard work with our clients to develop complete IVD solutions with our specialist knowledge of sensing technologies, fluid handling/microfluidics, materials science, and thermal control.
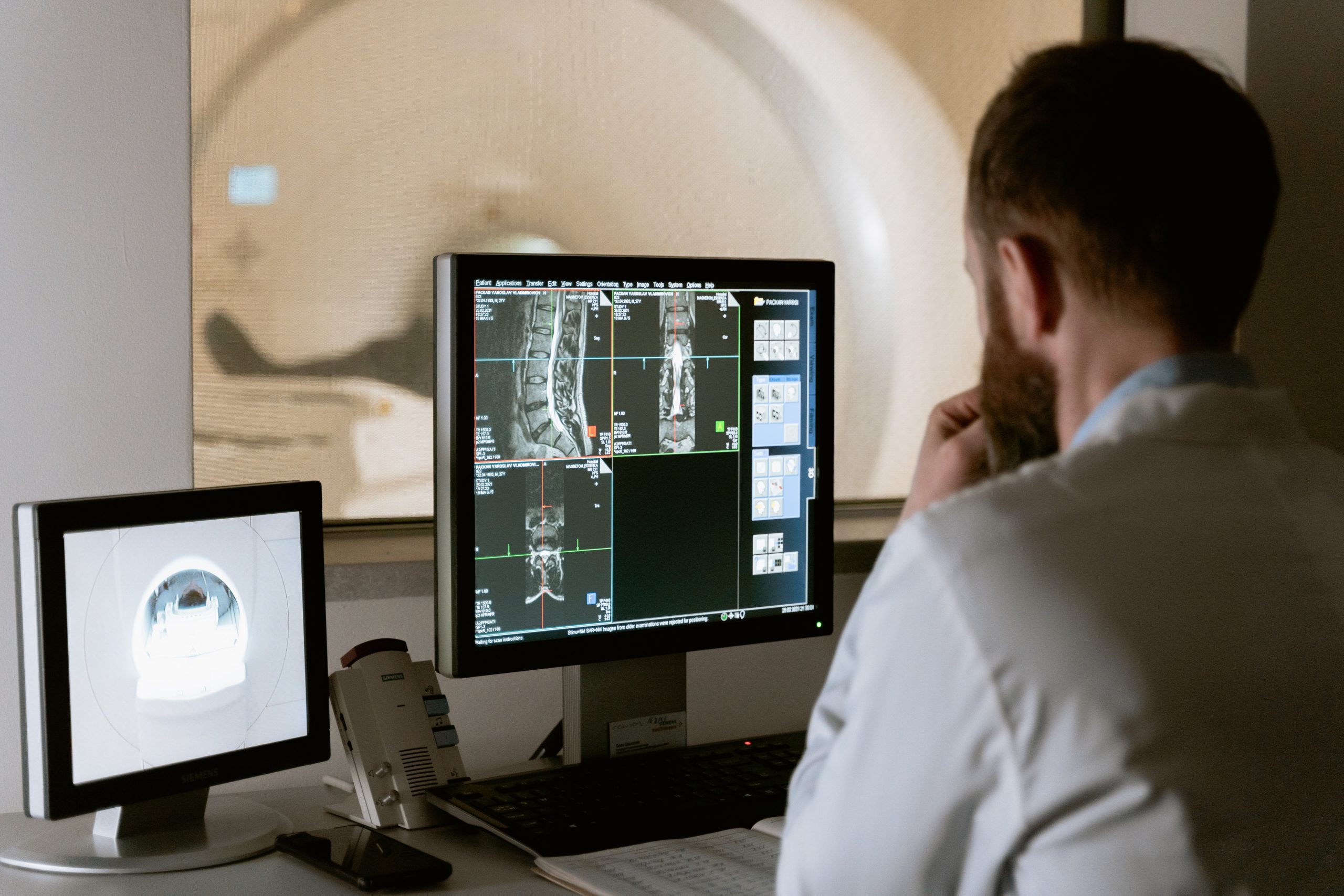
Imaging
Developing complex machines like MRI scanners, Ultrasound devices or even nuclear PET scanners takes time and considerable outlay. Therefore, it’s vital to get the design right, first time around.

On body diagnostics
Our extensive experience developing on body drug delivery devices translates well to the considerations needed of wearable diagnostics, and our team’s capabilities in usability engineering and industrial design ensure that all of our design put the user first.
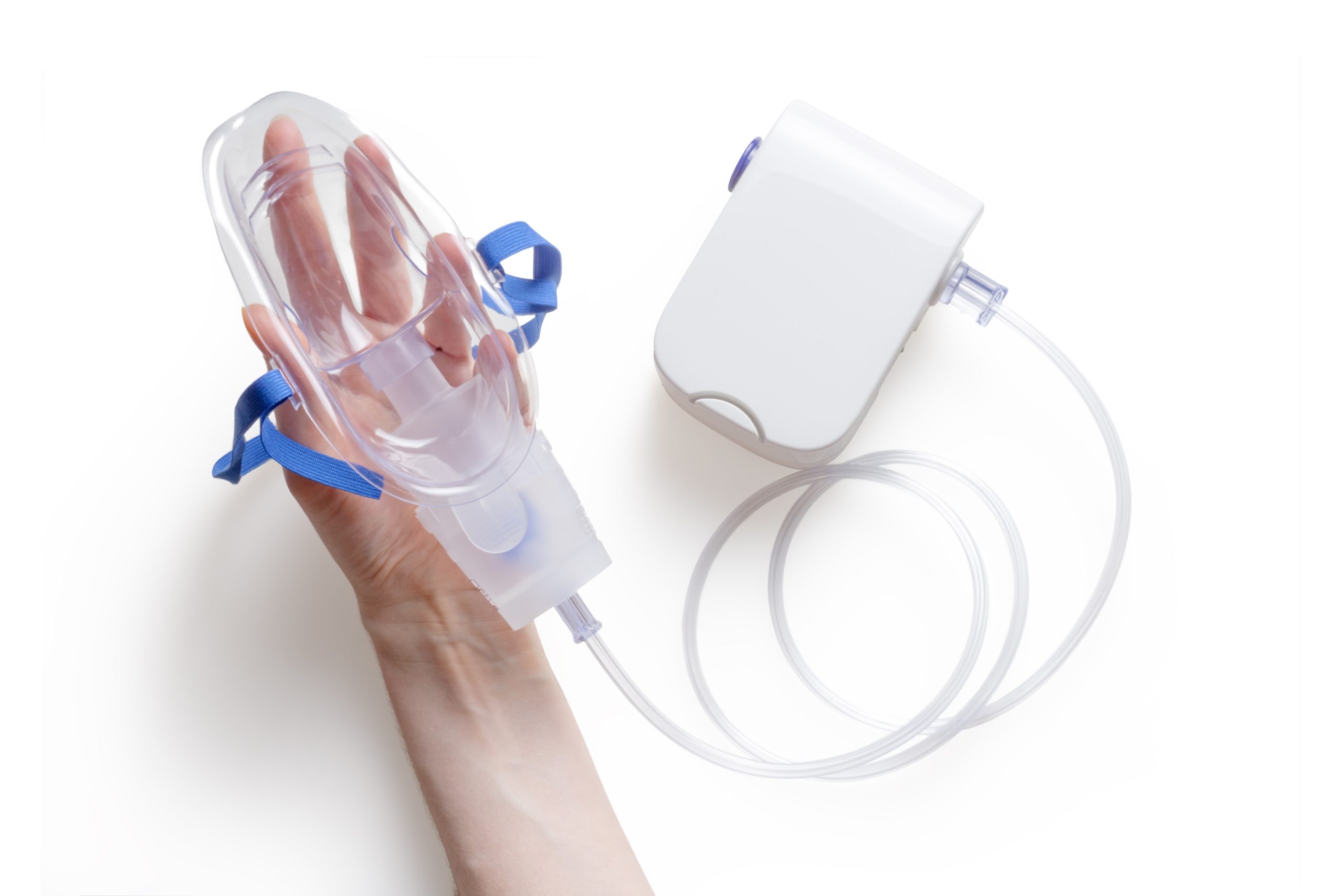
Respiratory diagnostics
Our engineers and scientists have the capabilities required to develop homecare devices, such as FeNO, spirometer and sleep monitoring technologies, where it is necessary to understand the science of the sensors as well as good engineering design and volume production requirements.
ISO 13485 Certified
All of our projects are managed with an ISO 13485 certified quality system, so quality is a habit for our team.
Our Quality Management System allows us to customise processes to fit our clients’ internal systems.