Bubble-Wrapped Remedies: How Lipid-Encased Therapeutics Could Transform Inhalation
30 April 2024
Introduction
In a post-COVID-19 era we have witnessed a notable shift in vaccine development with the advent of lipid nanoparticle (LNP) vaccines, representing a groundbreaking approach to immunization, opening new avenues for developing vaccines against various pathogens and reshaping shaping the future of preventive medicine. LNPs offer a means of “bubble-wrapping” therapeutic cargo, protecting it from degradation and facilitating their internalization into cells. Additionally, RNA carrying LNPs provide a unique opportunity to treat respiratory diseases resultant from inherited anomalies, utilising techniques like gene editing, silencing or replacement therapies to treat the root cause of the diseases [1].
The systemic delivery of LNP therapies via intravenous (IV) injection has significant challenges for pulmonary disorders, one being LNPs getting coated with apolipoprotein E (ApoE) protein in blood plasma. This ApoE coating causes LNP to be preferentially delivered to the liver rather than desired pulmonary tissues. Such challenges in IV injection of pulmonary therapies make inhalation a promising avenue for LNP delivery. However, the delivery of lipid-encapsulated therapies via inhalation is not without its own challenges with mucus [2] and lung surfactant [1] impacting reducing efficacy of LNP therapeutic delivery to target cells.
This article aims to shed light on some of the intricacies that lipid-encapsulated therapeutics bring to the realm of inhalation therapies, and some considerations on how the type of inhalation device selected may help mitigate issues around formulation stability and efficient delivery to target cells
Nano cargo ships: What is Lipid Nanoparticle Encapsulation?
Lipid nanoparticles (LNPs) are tiny particles typically ranging from 10 to 200 nanometres in diameter. These nanoparticles can be designed to target specific tissues or cells, release their cargo in response to specific triggers (such as pH or enzymes), and improve the bioavailability and efficacy of therapeutic compounds.
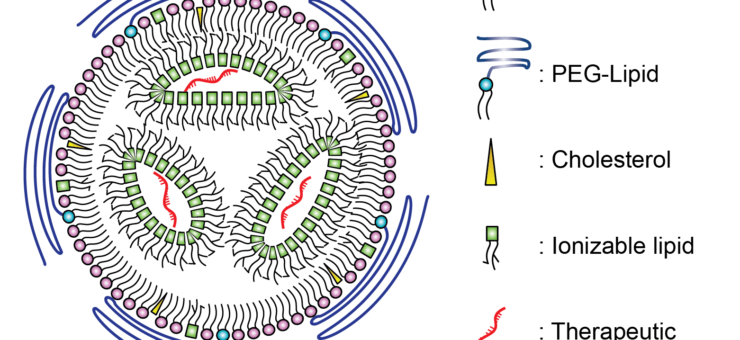
Lipid nanoparticle can be used to encapsulate therapeutic compounds, such as drugs or genetic material (like RNA or DNA). The LNP provides a protective shell for nucleic acids and other therapeutic cargos, as illustrated in Figure 1, preventing enzymatic degradation until the cargo is successfully delivered to the target cells [3]. The successful delivery of the nucleic acid cargo into the target cell is known as “transfection”.
Lipid nanoparticle encapsulation can be used for delivering a variety of therapeutics, including small molecule drugs, nucleic acids (such as DNA, RNA, siRNA), vaccines, proteins, peptides, imaging agents, and gene editing tools. The approach has applications not only for drug delivery and gene therapy, but also in diagnostics and imaging. Lipid nanoparticle encapsulation can enable the development of more effective and targeted therapies for various diseases and conditions, offering potential improvements in efficacy, safety, and patient outcomes.
Shear Brilliance: Mitigating the impact of shear stress on LNPs
Nebulisers and soft-mist inhalers aerosolise liquid formulations, generating a fine mist which the patient inhales to achieve direct delivery of therapeutics to the respiratory tract. The aerosolization process may subject the LNPs to high shear forces, especially during nebulization or when passing through narrow orifices. These forces can cause structural alterations or complete rupture of the lipid bilayer, leading to the premature release of encapsulated agents and, consequently, a significant loss of efficacy [4].
Nebulization mechanisms
To mitigate the impact of shear forces on LNP rupture and loss of efficacy, extensive research is underway into shear-reducing aerosolization techniques. Vibrating mesh nebulizers (Figure 2a) appear to be a more suitable method of gene therapy delivery compared to jet and ultrasonic nebulizers [4]. Conventional jet nebuliser designs often require a baffle in the aerosolization mechanism, creating large shear forces in the aerosolized product as it exits the nozzle. Instead, vibrating mesh nebulisers utilise a microscopic mesh vibrating at ultrasonic frequencies to create a fine mist from liquid medications. The absence of baffles within the vibrating mesh nebulisers reduces shear stress and damage to the LNPs and their cargo [5]. Future vibrating mesh nebuliser technologies will likely incorporate bespoke mesh technologies tailored for specific drugs and therapies, optimizing drug delivery for to the particular formulation of LNP encapsulated therapies.
In addition to the established nebulizer aerosolization mechanisms, two further mechanisms have been employed in soft-mist inhalers (SMIs): impinging jets (Respimat) and Rayleigh breakup (Medspray). A study comparing three nebulization techniques including vibrating mesh, impinging jets (Figure 2b) and a novel design employing Rayleigh breakup (Figure 2c), found that reducing the energy level required to aerosolize the formula is critical to safeguarding the integrity of the LNP and cargo [6]. The Rayleigh breakup mechanism requires much lower energy input to aerosolize the formula (2 J/g verses >20 J/g for other mechanisms), and hence was found to be the most successful. However, the nanoscale pores required may present manufacturing and maintenance challenges.
Lipid composition optimization
The optimization of lipid composition for increased resilience and developing novel formulations with inherent protective properties against mechanical stress is also a strategy being investigated. A recent study showed optimising LNP composition could improve mRNA transfection after aerosolization via a mesh nebuliser [7], by switching the ionizable lipid for a more saturated variant. This resulted in the aerosolized LNP being more effective at transfecting gene therapies to lung tissues in mouse models.
The developments in nebulization technology and optimization of lipid composition to increase resilience against shear suggest that liquid formulations and their accompanying inhaler devices will remain relevant in the future development of inhaled LNP drugs.
Powder Pioneers: Using LNPs in Dry Powder Inhalers
In addition to the challenge of shear forces, formulation in a liquid state increases the tendency of premature protein drug degradation during storage. The alternative to liquid nebulization is to first formulate as a dry powder and deliver using a dry-powder inhaler (DPI). DPIs have convenience benefits compared to nebulizers, being generally smaller and more portable, as well as cheaper. Compared to pMDIs, they do not require a propellant, which needs to be compatible with the drug.
Spray-drying is a common method employed for producing therapeutics as powders. The lipid solution containing the active pharmaceutical ingredient is atomized into fine droplets using a nozzle. These droplets are dried rapidly in a heated chamber, resulting in the formation of solid LNPs encapsulating the drug, suitable for pulmonary delivery through DPIs. Such powdered LNP formulations have been shown to be able to achieve low residual moisture levels (below 0.5%). When inhaled the dry powders form particle sized between 1 – 5 µm, ideal to be deeply inhaled into the lung and deposited in the alveolar region. Importantly, recent studies have shown sprayed dried LNPs retain their capability for transfection [8] and can be utilised to deliver gene therapies. Advanced drying techniques are also being developed, with one study preparing siRNA-encapsulated solid LNPS by thin-film freeze-drying showing promising results [9].
Studies have shown that the optimization of the excipient, the inactive vehicle of the LNP with the dry powder formulation, can significantly improve formulation characteristics. The combination of both mannitol and leucine has been demonstrated to create much smoother and spherical LNPs when observed through SEM imaging, and the addition of ethanol in the inlet feed aided in reducing particle size of the DPP [10]. Historically the choice of excipients approved for inhalation has been limited, with lactose and magnesium stearate being by far the most widely compounds used in inhaled drug products [11]. Diversification in approved excipient compounds will aid the future development of stable inhaled LNP therapeutics [12].
Puffing progress: Future considerations for LNP in inhalation devices
Lipid nanoparticle (LNP) therapeutics and inhalers are poised to drive innovation in pulmonary drug delivery, enhancing efficacy, safety, and patient experience. As highlighted in this article, one method of advancement lies in the refinement of LNP formulations to optimize their physicochemical properties for targeted lung delivery. This involves tailoring lipid composition, surface modifications, and encapsulation strategies to improve stability, bioavailability, and cellular uptake within the lungs. In parallel, advances in nanotechnology, such as the development of functionalized nanoparticles for targeted drug delivery and imaging, hold promise for synergistic approaches to disease diagnosis and treatment. By combining LNP-based therapeutics with imaging agents or nanoparticles, clinicians can gain insights into disease progression, monitor treatment response, and tailor therapy for optimal outcomes.
The integration of innovative technologies in the inhalation devices is also promising, for example the incorporation of microfluidics architectures. A recent study demonstrated a microfluidic system for producing aerosolized nanoparticles for inhaled mRNA therapy [13]. The particles were generated by individual microfluidic nozzles for precise droplet ejection, allowing for the low shear generation of droplets with minimal LNP disruption, aggregation or cargo leakage.
Overall, the future of LNP therapeutics and inhalers lies in multidisciplinary collaboration, technological innovation, and a patient-centric approach to respiratory care. By harnessing the potential of LNPs and inhalation devices, researchers and clinicians can revolutionize the management of lung diseases, improving quality of life and clinical outcomes for patients worldwide.
Written by Ethan Miller and Heather Jameson and featured in On Drug Delivery
References:
- Guilia Kassab, Katie Doran, Yulin Mo and Gang Zheng. 2023. “Inhalable Gene Therapy and the Lung Surfactant Problem.” Nanoletters 10099-10102.
- Jeonghwan Kim, Antony Jozic, Yuxin Lin, Yulia Eygeris, Elissa Bloom, Xiaochen Tan, Christopher Acosta, Kelvin D. MacDonald, Kevin Welsher, and Gaurav Sahay. 2022. “Engineering Lipid Nanoparticles for Enhanced Intracellular Delivery of mRNA through Inhalation.” ACS Nano 14792–14806.
- Albertsen, Camilla Hald, Jayesh Kulkarni, Dominik Witzigmann, Marianne Lind, Karsten Petersson, and Jen Simonsen. 2022. “The role of lipid components in lipid nanoparticles for vaccines and gene therapy.” Advanced Drug Delivery Reviews 114416.
- Eugene Arulmuthu, David Williams, Helen Baldascini, Henk Versteeg, Mike Hoare. 2006. “Studies on Aerosol Delivery of Plasmid DNA Using a Mesh Nebulizer.” Biotechnology and Bioengineering 939-955
- Sean McCarthy, Héctor González, Brendan Higgins. 2020. “Future Trends in Nebulized Therapies for Pulmonary Disease.” Journal of personalized medicine 37.
- van Rijn CJM, Vlaming KE, Bem RA, Dekker RJ, Poortinga A, Breit T, van Leeuwen S, Ensink WA, van Wijnbergen K, van Hamme JL, Bonn D, Geijtenbeek TBH. Low energy nebulization preserves integrity of SARS-CoV-2 mRNA vaccines for respiratory delivery. Sci Rep. 2023 May 31;13(1):8851
- Mae Lewis, Melissa Soto, Esther Maier, Steven Wulfe, Sandy Bakheet, Hannah Obregon, Debadyuti Ghosh. 2023. “Optimization of ionizable lipids for aerosolizable mRNA lipid nanoparticles.” Bioengineering & Translational Medicine 10580.
- Christoph M Zimmermann, Domizia Baldassi, Karen Chan, Nathan Adams, Alina Neumann, Diana Porras-Gonzalez, Xin Wei, Nikolaus Kneidinger, Mircea Gabriel Stoleriu, Gerald Burgstaller, Dominik Witzigmann, Paola Luciani, Olivia Merkel. 2022. “Spray drying siRNA-lipid nanoparticles for dry powder pulmonary delivery.” Journal of Controlled Release 137-150.
- Jie-Liang Wang, Mahmoud S. Hanafy, Haiyue Xu, Jasmim Leal, Yufeng Zhai, Debadyuti Ghosh, Robert O. Williams III, Hugh David Charles Smyth, Zhengrong Cui. 2021. “Aerosolizable siRNA-encapsulated solid lipid nanoparticles prepared by thin-film freeze-drying for potential pulmonary delivery”, International Journal of Pharmaceutics, Volume 596
- Ashish Sarode, Priyal Patel, Natalia Vargas-Montoya, Ayed Allawzi, Alisa Zhilin Roth, Saswata Karmakar, Lianne Boeglin, Hongfeng Deng, Shrirang Karve, Frank DeRosa. 2024. “Inhalable dry powder product (DPP) of mRNA lipid nanoparticles (LNPs) for pulmonary delivery.” Drug Delivery and Translational Research 360–372
- Manufacturing Chemist 8-May-2021 “Accelerating excipient development for inhalable dosage forms with QbD” accessed 20-March-2024 { https://manufacturingchemist.com/accelerating-excipient-development-for-inhalable-dosage-forms-with-qbd-176464#:~:text=The%20palette%20of%20approved%20excipients,used%20in%20inhaled%20drug%20products.}
- Leong EWX, Ge R. Lipid Nanoparticles as Delivery Vehicles for Inhaled Therapeutics. Biomedicines. 2022 Sep 2;10(9):2179
- Jeonghwan Kim, Antony Jozic, Elissa Bloom, Brian Jones, Michael Marra, Namratha Turuvekere Vittala Murthy, Yulia Eygeris, Gaurav Sahay. 2024. “Microfluidic platform enables shear-less aerosolization of lipid nanoparticles for messenger RNA inhalation.” bioRxiv Preprint.